HRPP: Metrics
The Purdue Human Research Protection Program (HRPP) and associated Institutional Review Board (IRB) strive to assist the research community on the application and review processes associated with human subjects research.
The Cayuse IRB protocol review system allows the HRPP to provide the research community with meaningful metrics to establish mutual expectations between researchers and reviewers. We will continue to provide new metrics as they become available.
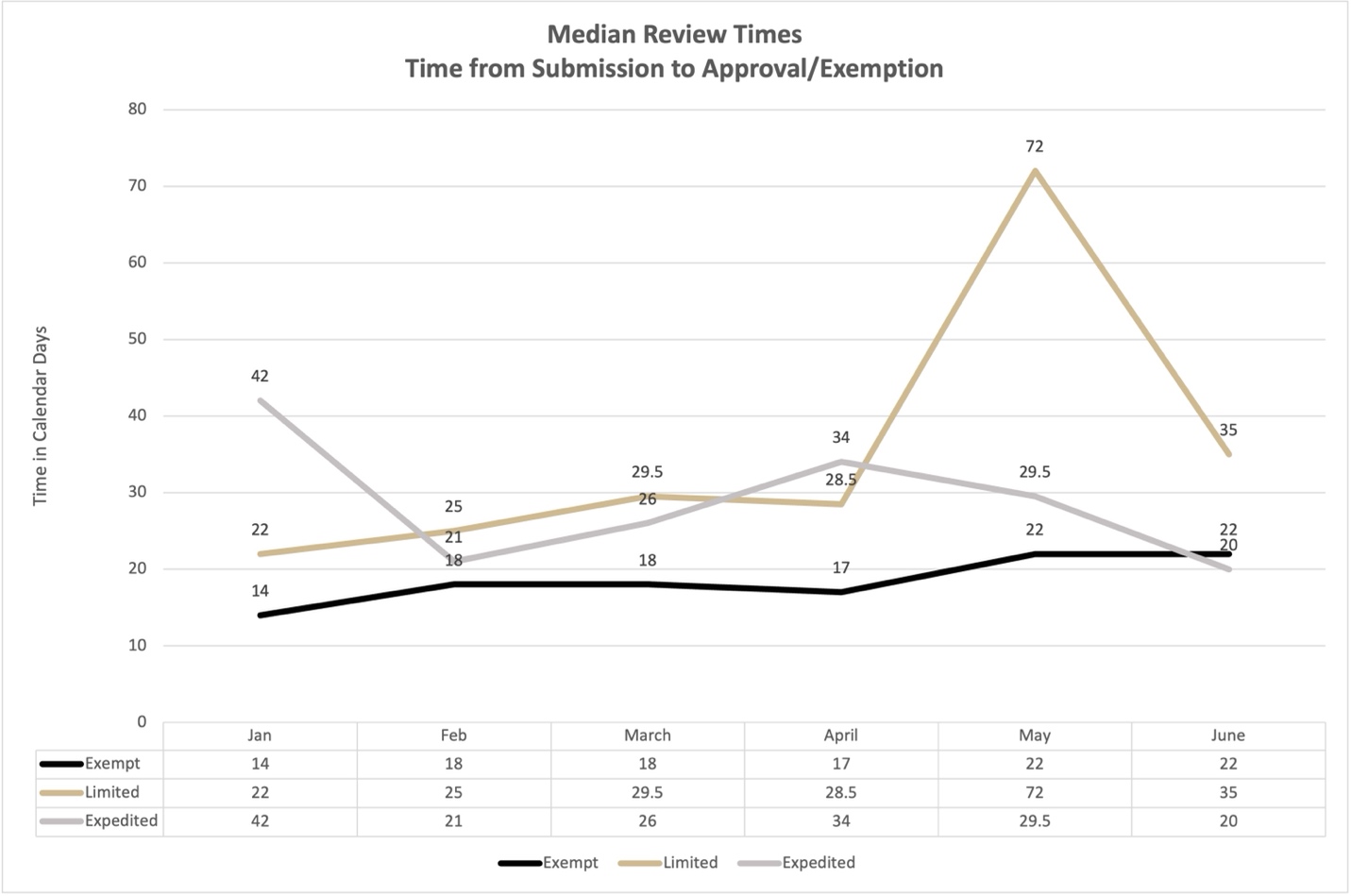
Review times are reported in three-month intervals expressed as median calendar days. A review begins when a new protocol is certified by the Principal Investigator and ends when a final determination letter is issued by HRPP/IRB. The processing includes all of the following:
- Verification of personnel and training – return if training is incomplete
- Pre-review by a protocol analyst
- Review by an IRB member or designated staff member
- Response to revisions (both by the HRPP and investigators)
- Processing the final decision and determination letter
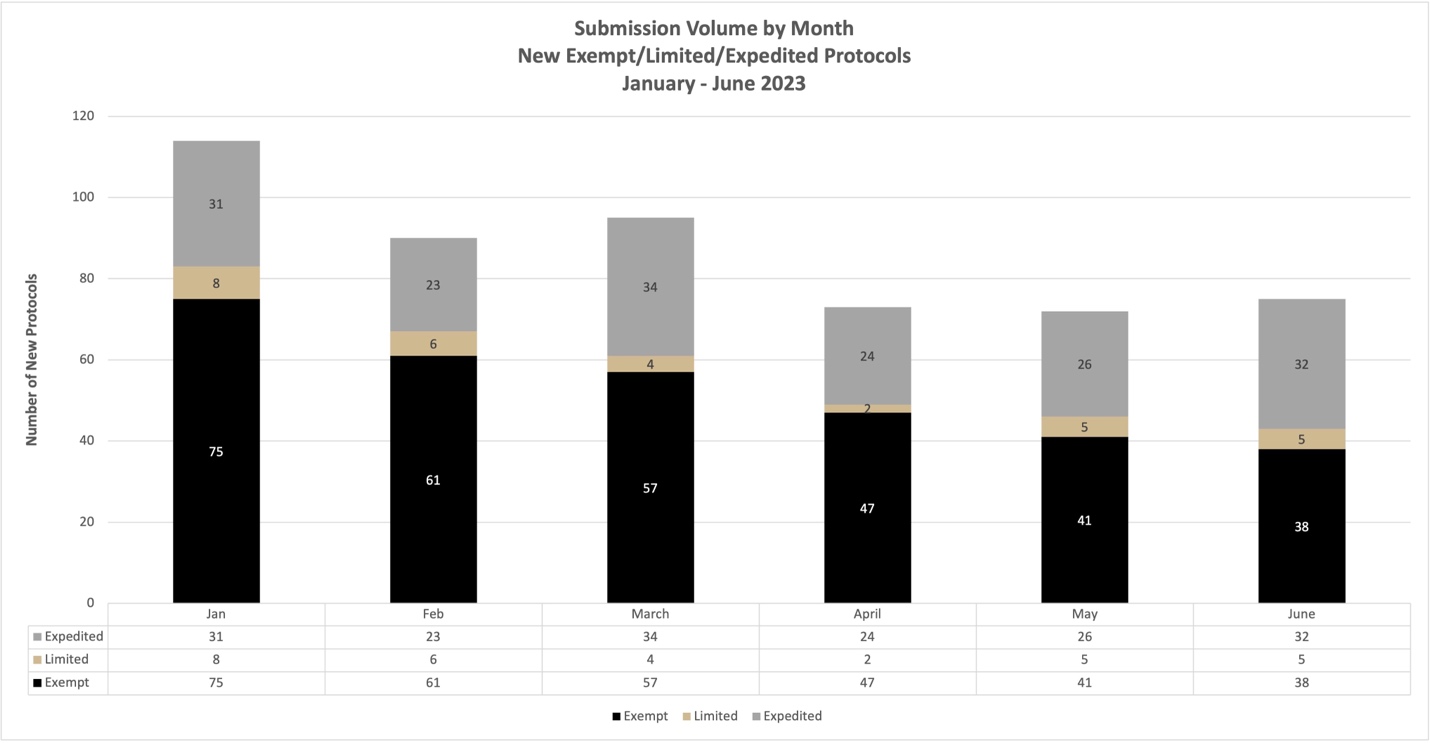
January–June 2023
New Cayuse Submissions Submission to Approval (Does not include renewals, modifications, or protocols pending revisions.)
| Number of Protocols | Median Review Days | Min. Review Days | Max. Review Days | |
|---|---|---|---|---|
| Exemptions | 319 | 18 | 0 | 193 |
| Limited Review | 30 | 35 | 5 | 136 |
| Expedited Review | 170 | 27 | 0 | 157 |
| Total | 519 | |||
January–June 2022
New Cayuse Submissions Submission to Approval (Does not include renewals, modifications, or protocols pending revisions.)
| Number of Protocols | Median Review Days | Min. Review Days | Max. Review Days | |
|---|---|---|---|---|
| Exemptions | 233 | 12 | 0 | 261 |
| Limited Review | 87 | 15 | 2 | 108 |
| Expedited Review | 142 | 28.5 | 0 | 426 |
| Total | 462 | |||
The convened (full) IRB meets twice per month. Protocols undergoing convened IRB review require a quorum of the board to review and vote. Please see meeting dates at https://www.irb.purdue.edu/irbs/meeting-dates.php.
January–June 2023
New Cayuse Submissions Submission to Approval (Does not include renewals, modifications, or protocols pending revisions.)
| Number of New Protocols | Median Review Days | Min. Review Days | Max. Review Days | |
|---|---|---|---|---|
| Full Board Review | 9 | 64 | 40 | 124 |
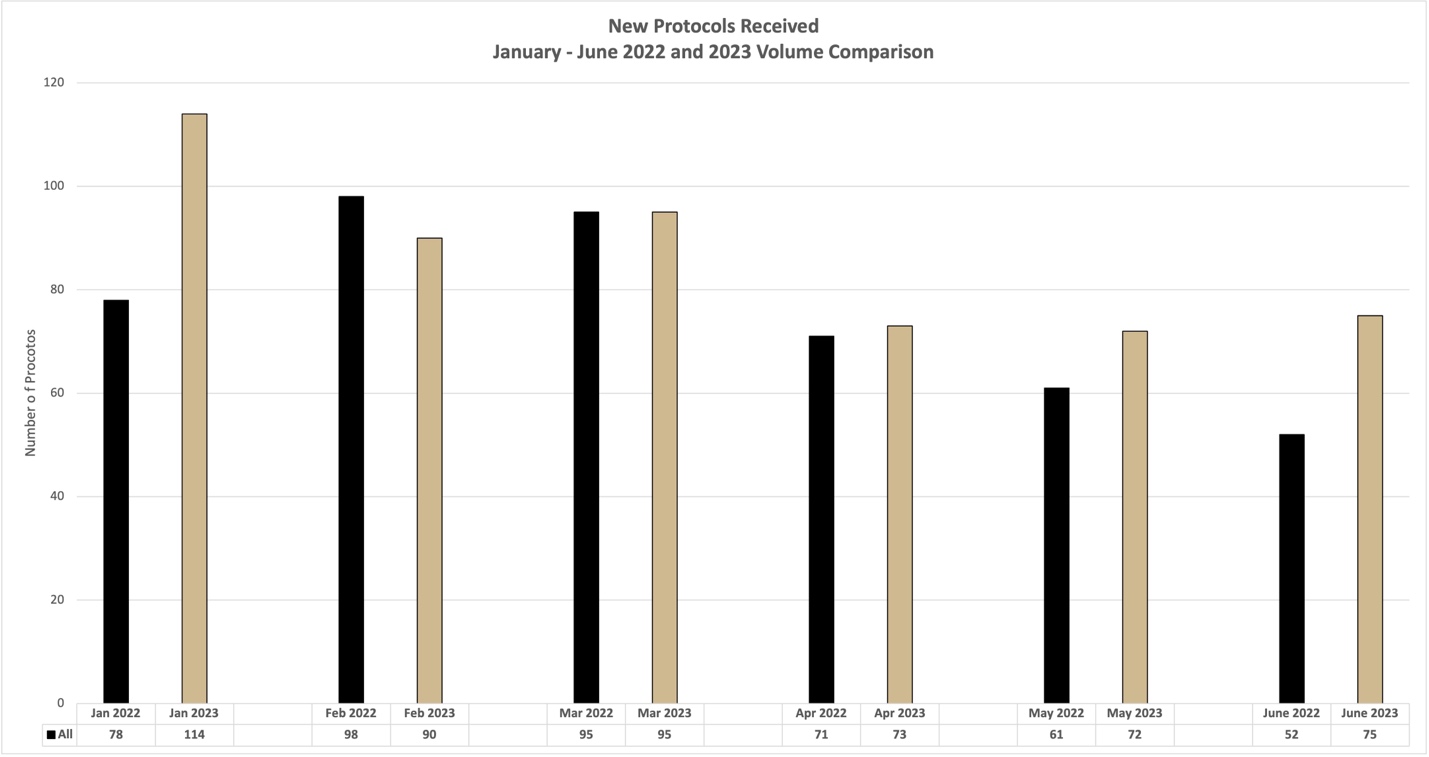
Further, a month to month summary of volume and review times is available for guidance. We recommend keeping these times and volume patterns in mind when submitting protocols for review. Thank you!
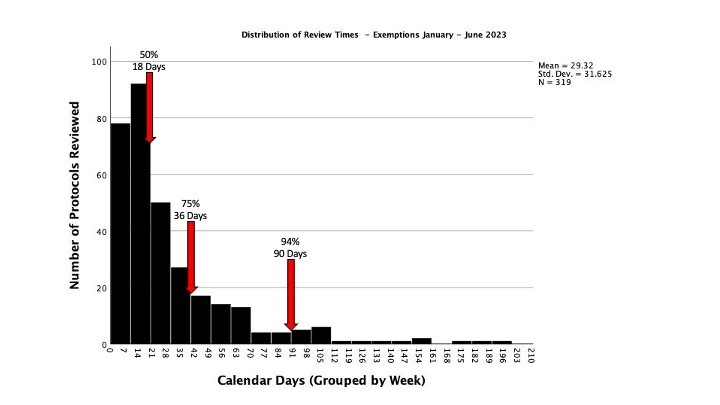
Finally, because the highest volume of HRPP/IRB reviews fall into Exempt categories, the distribution associated with these reviews is specifically outlined below. The arrows highlight the percentage of our exempt workload completed in this number of calendar days. This number is recorded in calendar days (with weekends and holidays) and reflects all time from submission to exemption including revisions requested from the research team. Note that protocols with revisions outstanding for longer than 90 days may be withdrawn.