Clinical Trials - Quick Reference
CITI New User Registration for Good Clinical Practice (GCP) Training
- Visit citiprogram.org
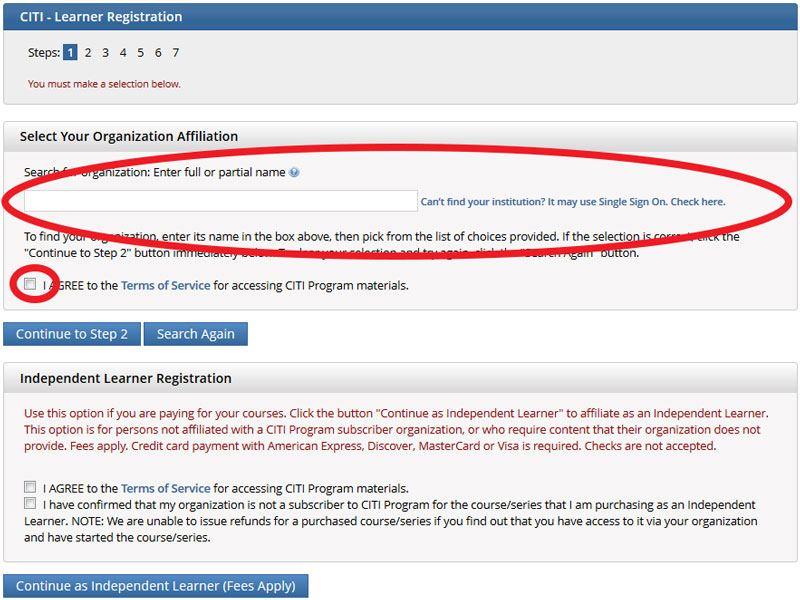
- New users must click on the “Register” button located in the blue box to the right of the homepage. The system is administered externally and is NOT directly connected to your Purdue Career account. If you have already created a login/password for the CITI system, please log in.
- Users with current login information will need to login to access the main menu and click “Add a Course or Update a Learner Group.” You must list your institutional affiliation with Purdue University. If you are not a new user, login and skip to step 8 of these instructions.
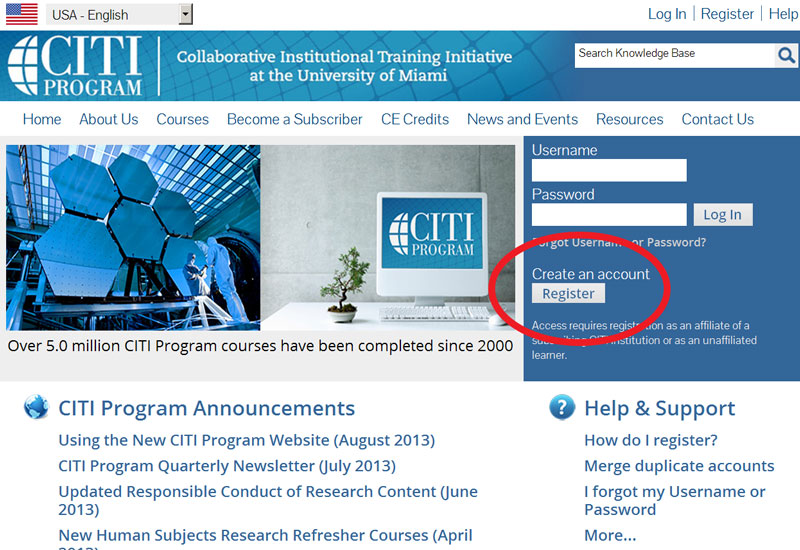
- Select the box under Search for Organization and type in Purdue University. Check mark the box that states “I agree to the Terms of Service for accessing CITI Program materials.”
- Note: If you do not select Purdue University, you will not complete the correct courses or have the proper documentation.
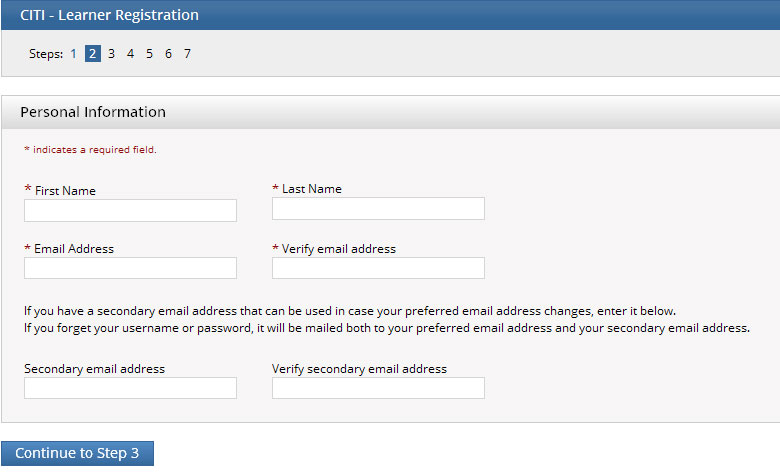
- Enter your name and email address. Please provide the name on file with Purdue University such that your training can be easily verified. It is also best to use your Purdue e-mail address.
- Choose a username and password for your account. Passwords are case sensitive. During this step you will also select and answer a security question.
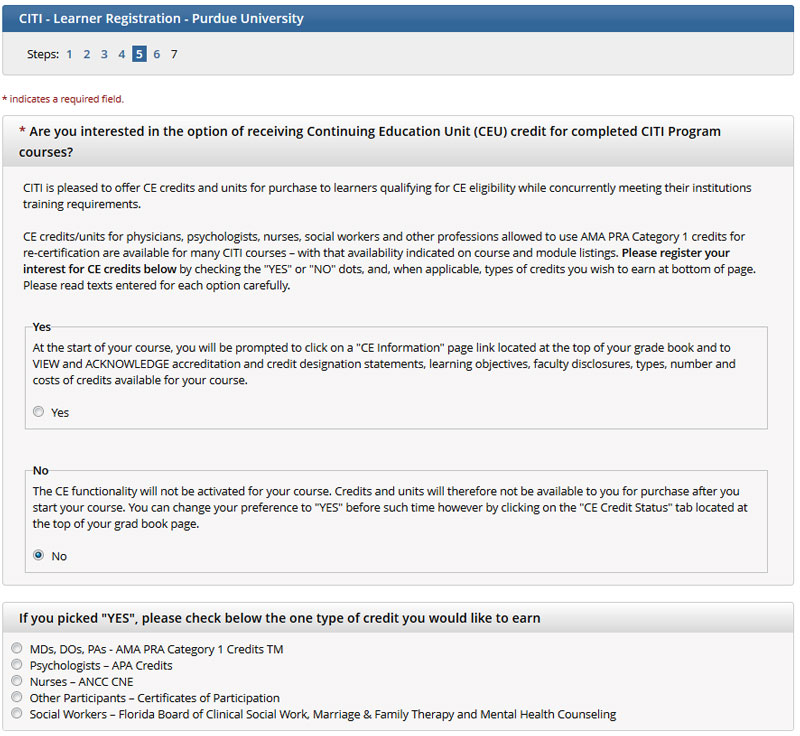
- Answer “No” regarding professional continuing education requirements. (Not applicable). There is no cost to this course for Purdue University employees and students. Also, answer if you are interested in participating in research surveys given by CITI at a later date. This answer is based on your personal preference.
- Complete the required demographic questions.
- Enroll in CITI Program courses by choosing your learner group.
- The group for the curriculum below apply only to Good Clinical Practices (GCP) Training.
- Scroll to the course on Good Clinical Practices (GCP). Complete the training labeled “Initial Training” to include GCP training modules on clinical trials with drugs, devices and biologics.
- GCP for Clinical Trials with Investigational Drugs and Biologics (ICH Focus) (ID: 127465)
- GCP for Clinical Investigations of Devices (ID: 127466)
- Click the “Complete Registration” button and finalize Registration.
- Your learner account registration is now complete. You will receive an account validation email to the address provided in Step 3.
- Complete the course and print or electronically store your completion certificate.

